Clinical indications
Postnatal
Children or adults with unexplained disease symptoms (Peripheral blood DNA) Prenatal
Prenatal
Miscarriage products of conceptions (chorionic villus DNA)
Fetal ultrasound structural abnormalities (amniocyte DNA)
High risk pregnancies (advanced maternal age, soft ultrasound marker)
Technology
Wet lab
Patent PCR-free library construction technology
Low input: 10-50ng DNA
Detection of aneuploidy, large fragment deletion/duplication, whole genome CNVs (>100kb) chromosome mosaicism (>10%)
Dry lab (Bioinformatics)
CNV analysis system
Case sharing – apoblema testing
Clinical information:
31 yrs, induced labor one time, a biochemical pregnancy, six times of embryo arrest. She did such test at 7+3 weeks, no fetal buds or fetal heart.
Test results:
seq [hg19] dup(2)(q36.1q37.3)
chr2:g. 224740001_ 243020000dup
seq [hg19] del(8)(q24.23q24.3)
chr8:g. 138800001_146300000del
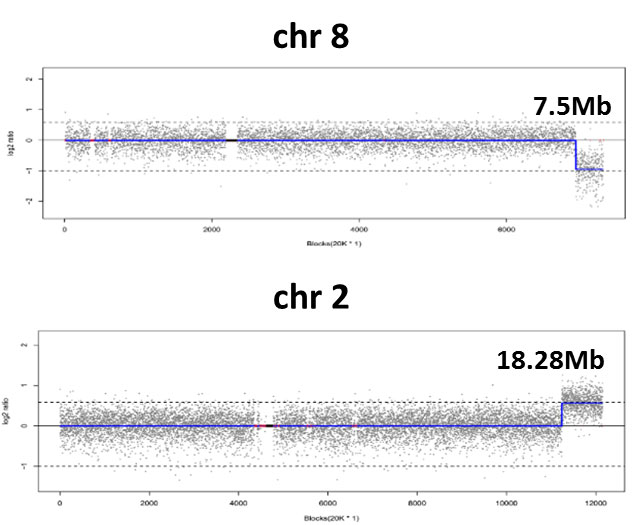
CNV-seq identified terminal deletion/duplication events at the end of chromosomes 2 and 8, indicating an balanced translocation.
Follow up FISH verified that the husband had t(2;8)(q36.1;q24.23) balanced translocation. The couple choose PGT and they successfully achieved a healthy baby.
Why choose Berry Genomics for CNV-Seq
Low cost
Integrated professional teams for sequencing, data analysis and reporting
Fast turnaround time
Reliable and accurate results equivalent to current array CGH and SNP arrays
The clinical significance of CNV-Seq
Can detect aneuploidy, CNVs (resolution 0.1Mb) and mosaicism (resolution) that are associated with known chromosome disease syndromes
Applicable to genetic diagnosis of preconception, prenatal and postnatal samples
Can identify a genetic cause of miscarriage samples
Reliable results can be obtained from rare samples or samples with low amounts of DNA


